Here is my third and final post on Boldrin and Levine’s book. I cover here the final two chapters and the pharma industry (chapter 9) and their conclusions (chapter 10). As lazy as usual, it is mostly some copy-pastes. As a reminder you can find here part 1 and part 2.
The Pharmaceutical Industry
The traditional model predicts that there should be many potential producers of a medicine, that the industry should be dynamically competitive, and therefore highly innovative with newcomers frequently challenging incumbents by means of innovative superior drugs. […] Some people esteem the pharmaceutical industry and some people despise it: there is little middle ground. The pharmaceutical industry is the poster-child of every intellectual monopoly supporter. It is the vivid example that, without the sheltering patents provide inventors with, the outpouring of new wonder drugs we have grown accustomed to would have not materialized, our life expectancies would be a lot shorter, and millions of people would have died of the diseases Big Pharma has instead managed to cure. In the opposite camp, Big Pharma is the scourge of humanity: a club of oligopolistic white men that, by controlling medicine around the globe and refusing to sell drugs at their marginal cost, are letting millions of poor people die. […] This sounds utterly complicated, so let us handle it with care and, for once, play the role of the wise fellows: in media stat virtus, et sanitas. […] How strong is the case for patents in pharmaceuticals? While Big Pharma is not necessarily the monster some depict, the case for patents in pharmaceuticals is a lot weaker than most people think. [Pages 242-243]
The authors make a long and interesting analysis of the history of the chemical industry with France and the UK blocking while Germany and Switzerland could innovate.
[Page 249] Here is how Murmann summarizes the main findings from his historical study of the European synthetic-dye industries during the 1857-1914 period. British and French synthetic dye firms that initially dominated the synthetic dye industry because of their patent positions but later lost their leadership positions are important cases in point. It appears that these firms failed to develop superior capabilities in production, marketing and management precisely because patents initially sheltered them from competition. German and Swiss firms, on the other hand, could not file for patents in their home markets and only those firms that developed superior capabilities survived the competitive home market. When the initial French and British patents expired, the leading German and Swiss firms entered the British and French market, capturing large portions of sales at the expense of the former leaders.
The authors also analyze Italy and India, which did not have until recently strong IP policies. “Interestingly though, we have not been able to find a single independent analyst claiming that the additional amount of pharmaceutical innovation patents may stimulate in the Indian industry, will be substantial and large enough to compensate for the other social costs. More to the point, the positive consequence of patent adoption in countries like India is, according to most analysts, a consequence of beneficial price discrimination. The argument goes as follows: monopoly power allows price discrimination – that is, the selling the same good for a high price to people valuing it a lot (usually people richer than average) and for a low price to people valuing it little (usually people poorer than average). Due to the absence of patent protection, there are very many new drugs that are not marketed in poor countries by their original producer, as the latter is not protected by reliable patents in that country.” [Page 253]
[Another] doubt comes from the following observation: if it were really true that imitating and “pirating” new drugs is that easy, absent patent protection local firms would be already producing and marketing such drugs in the country in question. [Page 254]
Pharma today
A few additional facts : the top 30 firms spend about twice as much in promotion and advertising as they do in R&D; and the top 30 are where private R&D expenditure is carried out, in the industry. Next we note that no more than 1/3 – more likely 1/4 – of new drug approvals are considered by the FDA to have therapeutic benefit over existing treatments, implying that, under the most generous hypotheses, only 25-30% of the total R&D expenditure goes toward new drugs. [Page 255]
Summing up and moving forward, here are the symptoms of the malaise we should investigate further.
– There is innovation, but not as much as one might think there is, given what we spend.
– Pharmaceutical innovation seems to cost a lot and marketing new drugs even more, which makes the final price for consumers very high and increasing.
– Some consumers are hurt more than others, even after the worldwide extension of patent protection. [Page 256]
Where do drugs come from? [Pages 257-260]
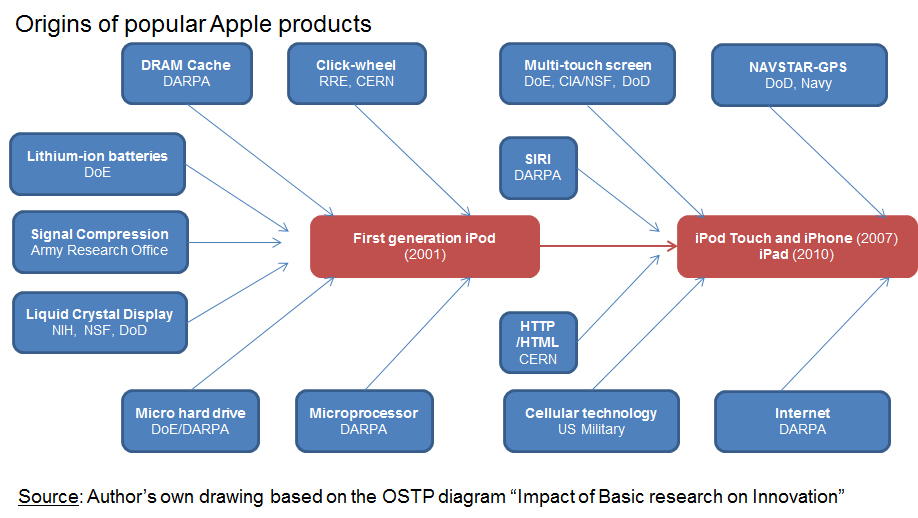
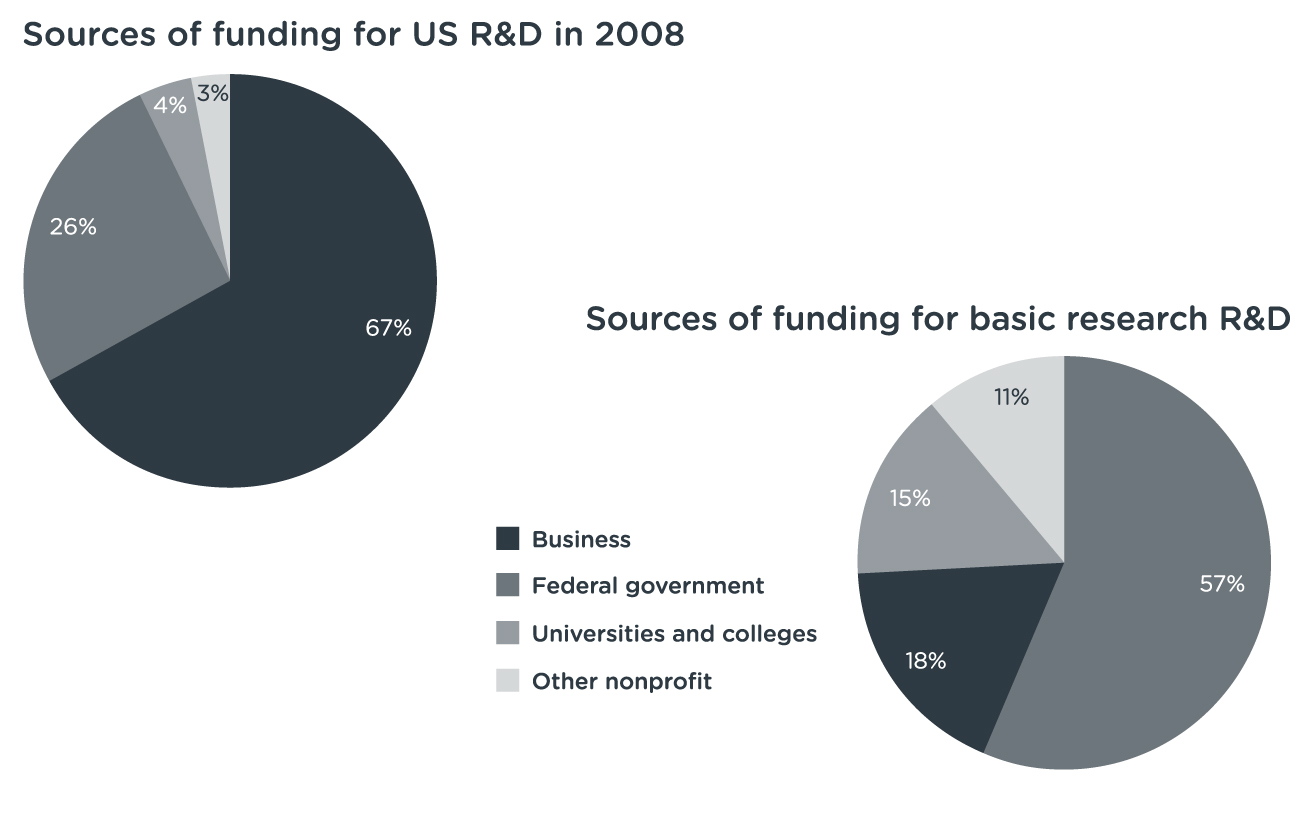
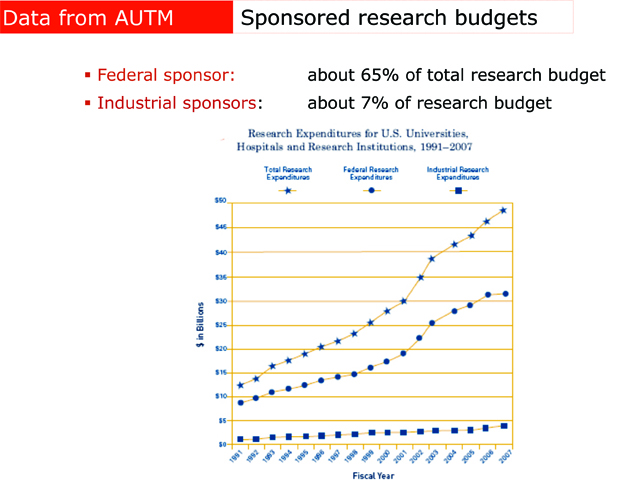
Useful new drugs seem to come in a growing percentage from small firms, startups and university laboratories. […]Next there is the not so small detail that most of those university laboratories are actually financed by public money, mostly federal money flowing through the NIH. The pharmaceutical industry is much less essential to medical research than their lobbyists might have you believe. In 1995, […] about $11.5 billion came from the government, with another $3.6 billion of academic research not funded by the feds. Industry spent about $10 billion. However, industry R&D is eligible for a tax credit of about 20%, so the government also picked up about $2 billion of the cost of “industry” research. […] In 2006, total was $57 billion while the NIH budget in the same year (the largest but by no means the only source of public funding for biomedical research) reached $28.5 bn.[…] it is wise to remember that the modern “cocktail” that is used to treat HIV was not invented by a large pharmaceutical company. It was invented by an academic researcher: Dr. David Ho.
It is a fact that, without the strong incentive the prospect of a successful patent induces, those researchers would not be working as hard as they do. That is true, so let us think the issue through once again. We observe that, while the incentive to patent and commercialize their findings should have been increased by the Bayh-Dole act allowing patentability of such research results, there is no evidence whatsoever that, since 1980 when the act was passed, major medical scientific discoveries have been pouring out of American universities’ laboratories.
It therefore remains an open question: did patentability of basic biomedical innovations create an incentive for engaging in more socially valuable research projects and investigations? Which medical and pharmaceutical discoveries are truly fundamental and where do they come from?
Here are the selected fifteen most important medical milestones: Penicillin, x rays, tissue culture, ether (anaesthetic), chlorpromazine, public sanitation, germ theory, evidence based medicine, vaccines, the pill, computers, oral rehydration therapy, DNA structure, monoclonal antibody technology, smoking health risk. How many entries in this list were patented, or were due to some previous patent, or were obtained during a research project motivated by the desire to obtain a patent? Two: chlorpromazine and the pill. […] Now the “list of Top Pharmaceuticals”, these are the current pharmaceutical products selling the most worldwide, and there are 46 of them. Patents had pretty much nothing to do with the development of 20 among the 46 top selling drugs. […] Notice though that of these 26, 4 were discovered completely by chance and then, 2 were discovered in university labs before the Bayh-Dole Act was even conceived. Further, a few were simultaneously discovered by more than one company leading to long and expensive legal battles, however, the details are not relevant to our argument. The bootom line is more than half of the top selling medicines around the world do not owe their existence to pharmaceutical patents.
Rent-seeking and redundancy. [Pages 260-263]
The next question then is, if not in fundamental new medical discoveries, where does all that pharmaceutical R&D money go? […] 54% of FDA-approved drug applications involved drugs that contained active ingredients already in the market. […] 35% were products with new active ingredients, but only a portion of these drugs were judged to have sufficient clinical improvements over existing treatments to be granted priority status. In fact, only 238 out of 1035 drugs approved by the FDA contained new active ingredients and were given priority ratings on the base of their clinical performances. In other words, about 77% of what the FDA approves is “redundant” from the strictly medical point of view. […] Sad but ironically true, me-too or copycat drugs are largely the only available tool capable of inducing some kind of competition in an otherwise monopolized market. ..] The ironic aspect of me-too drugs, obviously, is that they are very expensive because of patent protection, and this cost we have brought upon ourselves for no good reason. […] Insofar as new drugs are replacements for drugs that already exist, they have little or no economic value in a world without patents – yet cost on the order of $800 million to bring to market because the existence of patents forces the producers to “invent something” the USPTO can pretend to be sufficiently different from the original, patented, drug.
Libraries have been written on the obvious connection between marketing and the lack of competition. The pharmaceutical industry is no exception to this rule, and the evidence Professor Sager, and many others, point to has a simple and clear explanation: because of generalized and ever extended patenting, large pharmaceutical companies have grown accustomed to operating like monopolies. Monopolies innovate as little as possible and only when forced to; in general they would rather spend time seeking rents via political protection while trying to sell at a high price their old refurbished products to the powerless consumers, via massive doses of advertising.
The authors finish with an economic analysis of the social cost and benefit of patents and without patents, and propose solution in the final chapter.
Conclusion: The bad, the Good, and the Ugly
Edith Penrose, concluded that “If we did not have a patent system, it would be irresponsible, on the basis of our present knowledge of its economic consequences, to recommend instituting one. But since we have had a patent system for a long time, it would be irresponsible, on the basis of our present knowledge, to recommend abolishing it
But the authors claim: “On the basis of the present knowledge” progressively but effectively abolishing intellectual property protection is the only socially responsible thing to do. […] A realistic view of intellectual monopoly is that it is a disease rather than a cure. It arises not from a principled effort to increase innovation, but from a noxious combination of medieval institutions – guilds, royal licenses, trade restrictions, religious and political censorship – and the rent-seeking behavior of would be monopolists seeking to fatten their purse at the expense of public prosperity. [Pages 277-78]
A myriad of other legal and informal institutions, business practices and professional skills have grown up around them and in symbiosis with them. Consequently, a sudden elimination of intellectual property laws may bring about collateral damages of an intolerable magnitude. Take for example the case of pharmaceuticals. Drugs are not only patented, they are also regulated by the government in a myriad of ways. Under the current system, to achieve FDA approval in the United States requires costly clinical trials – and the results of those trials must be made freely available to competitors. Certainly, abolishing patents and simultaneously requiring firms that conduct expensive clinical trials to make their results freely available to competitors, cannot be a good reform. Here patents can only be sensibly eliminated by simultaneously changing also the process by which the results of clinical trials are obtained, first, and, then, made available to the public and to competitors in particular. [Page 278]
The authors look at many intermediate solutions including deregulation, private contracts, subsidies, social norms but they are clearly convinced (and convincing) that an evolution is needed, even if they are pessimistic:
Where, today, is a software innovator to find safe haven from Microsoft’s lawyers? Where, tomorrow, will be the pharmaceutical companies that will challenge the patents of “big pharma” and produce drugs and vaccines for the millions dying in Africa and elsewhere? Where, today, are courageous publishers, committed to the idea that accumulated knowledge should be widely available, defending the Google Book Search initiative? Nowhere, as far as we can tell, and this is a bad omen for the times to come. The legal and political war between the innovators and the monopolists is a real one, and the innovators may not win as the forces of “Stay the Course” and “Do Nothing” are powerful, and on the rise. [Page 299]
Certainly the basic threat to prosperity and liberty can be resolved through sensible reform. But intellectual property is a cancer. The goal must be not merely to make the cancer more benign, but ultimately to get rid of it entirely. So, while we are skeptical of the idea of immediately and permanently eliminating intellectual monopoly – the long-term goal should be no less than a complete elimination. A phased reduction in the length of terms of both patents and copyrights would be the right place to start. By gradually reducing terms, it becomes possible to make the necessary adjustments – for example to FDA regulations, publishing techniques and practices, software development and distribution methods – while at the same time making a commitment to eventual elimination. [Page 300]